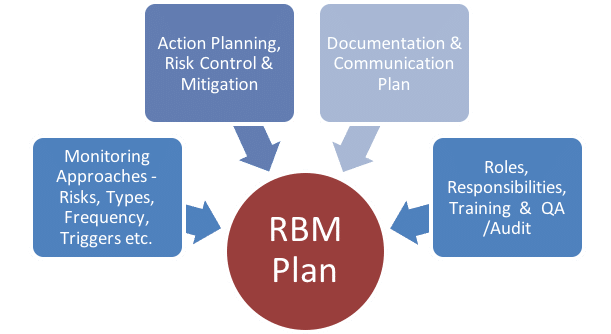
CRAB creates a central monitoring plan that can be tailored to meet your needs. CRAB provides Risk Based Monitoring and Centralized Monitoring as defined in the August 2013 guidance document issued by the FDA titled “Oversight of Clinical Investigations —A Risk-Based Approach to Monitoring.” Risk-Based Management, or RBM, is an innovative monitoring service performed by the collaboration of CRAB’s Data Management and Central Monitor staff and includes a risk-based assessment of the clinical trial’s complexity, endpoints and safety evaluations.
Our skilled and well-trained monitors can provide the following:
Monitoring Algorithm
Conducted remotely via a Source Document Portal (SDP) for source document verification confirming eligibility, consent process and accurate data submission on electronic case report forms. Sites are notified by email when a patient is enrolled and a list of expected source documents to upload into the SDP is also provided. Central monitors communicate with sites for any source/data discrepancies. Once review has been complete, a final review letter is provided to the site.
Troubleshooting within the Source Document Portal
Sites can contact our central monitors for any issues regarding source document expectations. Technical issues with accessing the SDP can also be resolved by our central monitoring team.